Nguyen Pham Thao Nhi, Nguyen Khanh Thuan, Nguyen Phuc Khanh, Nguyen Thanh Lam*
1. Introduction
Lumpy skin disease (LSD) is an OIE notifiable, vector-borne disease of cattle caused by lumpy skin disease virus (type strain, Neethling), which together with sheep and goat poxviruses, constitute the Capripoxvirus genus of the Poxviridae family. This virus does not cause disease in humans. Although the mortality rate is generally low, economic losses result from loss of condition, decreased milk production, abortions, infertility and damaged hides. The causative virus seems to be spread mainly by blood-feeding insects, such as certain species of flies and mosquitoes or ticks, and outbreaks can be widespread and difficult to control (Tuppurainen et al., 2015). LSD was first reported in Asia and the Pacific region in 2019 in northwest China, Bangladesh and India. During the northern summer of 2020, LSD has continued its spread across continental Asia with many members in south and southeast Asia. LSD outbreaks tend to be sporadic, depending upon animal movements, immune status, and wind and rainfall patterns affecting vector populations. The principal method of transmission is thought to be mechanical by arthropod vector.
2. Aetiology
2.1 Virus characteristics
The LSD virus (LSDV) belongs to the genus Capripoxvirus within the Poxviridae family and shares high antigenic similarities with the sheeppox virus (SPPV) and the goatpox virus (GTPV), two other members of this genus. While SPPV and GTPV serologically crossreact with LSDV, they do not cause disease in species other than their respective host (Roche et al., 2021).
Capripoxvirus is the most economically significant in the Poxviridae family affecting domestic ruminants in Africa and Asia. They are double-stranded DNA (dsDNA) viruses containing around 150 Kbp and are relatively large (230-260 nm). Their capsid or nucleocapsid is brick- or oval-shaped containing the genome and lateral bodies. There is extensive DNA cross-hybridization between species which accounts for serologic cross-reaction and cross-protection among members. The LSDV has enveloped DNA virus, with 151-Kbp genome and consists of a central coding region bounded by identical 2.4 kbp inverted terminal repeats and contains 156 putative genes. The virus encodes 30 homologues of poxviral proteins known to be structural or nonstructural which is antigenically and genetically closely related to SPPV and GTPV with nucleotide sequence identities of 96% between species. Although Capripoxviruses are generally considered to be host-specific, SPPV and GTPV strains can naturally or experimentally cross-infect and cause disease in both host species. In contrast, LSDV can experimentally infect sheep and goats, but no natural infection of sheep and goats with LSDV has been described so far (Mulatu and Feyisa, 2018).
Poxviruses are the only DNA viruses known to complete their replication cycle in the cytoplasm. In the cytoplasm, the dsDNA is used as a template for both mRNA production (for translation of proteins) and copies of the genome for progeny virions; viral enzymes largely mediate both processes. As the virions are large and complex, the mechanism associated with virion assembly is largely unknown. Virions are released from the cell by budding. Poxviridae families possess at least 10 major antigens with a common nucleoprotein antigen, which accounts for cross-reactivity among species. There are at least 10 viral enzymes contained within the virus particle, many of which function in nucleic acid metabolism and genome replication (Mulatu and Feyisa, 2018).
2.2 Virus classification
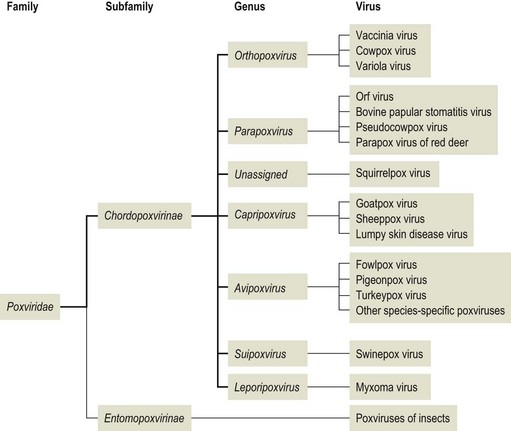
The family Poxviridae contains the largest viruses which are able to cause disease naturally in most domestic animals, except in dogs. It is divided into two subfamilies, Chordopoxvirinae, the poxviruses of vertebrates and Entomopoxvirinae, the poxviruses of insects (Markey et al., 2013).
2.3 Virus resistance
Lumpy skin disease virus is stable in the environment and may remain viable up to three months in dry scabs on skin, at least six months in dirty, shaded pens and infected tissue culture fluid stored at 4°C. Infected animals shed scabs from skin lesions and inside the scabs the virus may remain infectious for several months. LSDV survives in necrotic skin nodules for at least 39 days even dried out prior to sequestration and in air-dried hides at room temperature for at least 18 days. No studies have identified how long it takes for LSDV to lose infectivity in different environments. In addition, it is sensitive to sunlight and detergents containing lipid solvents, but in dark and humid environments, such as contaminated barns, the virus can survive for many years month (Roche et al., 2021).
Lumpy skin disease virus survives well within the pH range 6.3-8.3. It is highly susceptible to sunlight, high alkaline or acid pH; can be inactivated at 55º C for 2 h, 60º C for 1 h or 65º C for 30 min, or by most detergents such as sodium dodecyl sulfate and detergents containing lipid solvents; (2 percent) Virkon®, (2–3 percent) sodium hypochlorite, (20 percent) chloroform, (2 percent) phenol in 15 min, (1 percent) formalin, (1:33) iodine compounds, and (0.5 percent) quaternary ammonium compounds (Roche et al., 2021).
3. History of disease
The clinical syndrome of cellulite LSD was first described in Zambia (formerly Northern Rhodesia) in 1929 (Alexander et al., 1957). It was initially thought to be the result of poisoning or hypersensitivity to insect bites. Between 1943 and 1945, the cases occurred in Botswana (Bechuanaland), Zimbabwe (Southern Rhodesia) and the Republic of South Africa. The infectious nature of the disease was recognized at this time. A panzootic in South Africa, which lasted until 1949, affected some eight million cattle and consequently incurred enormous economic losses (Davies, 1991). From 1929 to 1986, the disease was limited to countries in sub-Saharan Africa. In May 1988, LSD was clinically recognized in the Suez Administration of Egypt. The disease spread locally in the summer of 1988 (Davies, 1991). During a period of 37 days between August and September 1989, fourteen of the seventeen dairy herds in Peduyim became infected with LSD. All of the cattle as well as small flocks of sheep and goats in the village were slaughtered (Yeruham et al., 1995).
4. Epidemiology
4.1 Geographic distribution
Lumpy skin disease virus was diagnosed for the first time in Zambia in 1929 and then reported in several regions of African countries (Weiss and Gard, 2013). The disease has spread progressively and extensively throughout Africa, the Middle East, Southeastern Europe, Central Asia, and more recently South Asia and China. Currently, the disease is endemic in several countries across Africa, parts of the Middle East (Iraq, Saudi Arabia, Syrian Arab Republic) and Turkey (EFSA, 2020).
July 2019 marked the first known introduction of LSD into South Asia, with Bangladesh officially reporting an outbreak. In August 2019, the disease appeared in India and western China, in the Xinjiang Uyghur Autonomous Region bordering Kazakhstan. In June 2020 LSD was again observed in China. Outbreak reports from other provinces, namely Fujian (1), Jiangxi (2), Guangdong (1), Anhui (1), Zhejiang (1) and Taiwan Province of China (34) followed, indicating the continued and widespread presence of the disease. This is supported by announcements on designation of control zones and implementation of LSD control measures also in Guangxi Autonomous Region and Yunnan Province. Although not yet officially reported to OIE, according to media reports LSD has spread to the southern part of India since January 2020 (Roche et al., 2021).
In Bangladesh, LSD had spread to all divisions by December 2019 and despite lack of official reports, several LSD type disease events are mentioned in media articles suggesting the disease is present in the country and had likely reached the northern most districts by March 2020. This hypothesis is supported by an unpublished study in Bangladesh evidencing LSD gene fragments through polymerase chain reaction (PCR) assay in cattle samples collected between July 2019 and January 2020. However, these findings were not reported through official channels. In June 2020 LSD affected Nepal for the first time with two outbreaks in the eastern part of the country. As of 18 September 2020, LSD continues to spread in the East and South Asia regions, with a number of outbreaks reported from seven provinces spanning South and East China, as well as reports from Nepal and India, and a recent confirmation of disease introduction in Bhutan.
The recent LSD introductions in Asia are of concern as India, China and Bangladesh have some of the world’s largest bovine populations. COVID-19 lockdown enforced in many Asian countries exacerbates already existing difficulties for veterinary services and laboratories trying to conduct timely outbreak investigation and disease diagnosis, which may result in delayed disease detection, reporting and implementation of control measures. There have been few risk assessments or risk models addressing introduction or spread of LSD in the past, these mainly targeting countries in Africa, Europe and Central Asia. In August 2019, the disease appeared in India and western China, in the Xinjiang Uyghur Autonomous Region bordering Kazakhstan. In June 2020 LSD was again observed in China (Roche et al., 2021).
4.2 Susceptible hosts
Lumpy skin disease is an infectious disease of cattle and Asian water buffalo (Bubalus bubalis). Breeds of Bos taurus with high milk production are more susceptible than African/Asian indigenous cattle (Bos indicus). The morbidity varies from 2% to 45% and is lower in Asian water buffaloes. Mortality in cattle is usually less than 10 percent, but can be higher in certain breeds, age groups or in high milk producing cows (Tuppurainen et al., 2017).
In wildlife, clinical LSD has been reported in an Arabian oryx (Oryx leucoryx), in springbok (Antidorcas marsupialis), and experimental infection could produce clinical signs in impala (Aepyceros melampus) and giraffe (Giraffa camelopardalis) and Thomson’s gazelle (Eudorcas thomsonii). Blue wildebeest (Connochaetes taurinus), black wildebeest (Connochaetes gnou), springbok, impala and eland (Taurotragus oryx) have tested positive for LSD antibodies in South Africa as have African buffalo (Syncerus caffer) in Kenya (Davies and Otema, 1981) and South Africa. However, no LSD case has ever been detected in these species in their natural habitat and the possible role of wild ruminants in LSD epidemiology is still unknown. Asia has unique wild species of genus Bos and Bubalus, some of which are listed by the Convention on International Trade in Endangered Species of Wild Fauna and Flora. Their susceptibility to LSDV is currently unknown(Roche et al., 2021).
4.3 Transmission
Lumpy skin disease is transmitted primarily mechanically by blood-feeding insects. Though no specific vector has been identified to date, mosquitoes (e.g. Culex mirificens and Aedes natrionus), biting flies (e.g. Stomoxys calcitrans and Biomyia fasciata) and male ticks (Riphicephalus appendiculatus and Amblyomma hebraeum) could play a role in the transmission of the virus. The importance of different 2 arthropod vectors is likely to vary in different areas depending on the abundance and feeding behavior of the vector (OIE, 2019). Other routes of spread are iatrogenic, through direct or indirect contact and artificial insemination. Infected bulls can excrete the virus in the semen (OIE, 2019). LSD virus is found in blood between 7 and 21 days after infection. Shedding of the virus in semen can last up to 42 days. Also, there is evidence of placental transmission of the virus.
In experimental studies, the persistence of LSDV was indicated in bovine semen by both PCR and virus isolation (Annandale et al., 2010; Givens, 2018; Irons et al., 2005). Also, semen caused the transmission of the virus to inseminated heifers (Annandale et al., 2014).
5. Pathogenesis
Sheeppox, goatpox, and lumpy skin disease are all systemic diseases, with cell-associated viremia preceding the appearance of lesions and marked lymphadenopathy. It is likely that blood monocytes are important in spreading virus to secondary sites of infection. Like most members of the subfamily Chordopoxviridae, Capripoxviruses exhibit a distinct tropism for keratinocytes. Skin lesions are characterized by hyperplasia and ballooning degeneration of keratinocytes of the stratum spinosum, formation of epidermal microvesicles, and infiltration of inflammatory cells into the dermis. In lumpy skin disease, epidermal microvesicles coalesce into large vesicles that quickly ulcerate. Nodular proliferative lesions can occur internally in severe sheeppox and goatpox, most notably in the lungs but also in the forestomachs, and less frequently in the liver, tongue, and kidneys. Lung lesions are markedly proliferative in nature, involving hyperplasia of type II pneumocytes and the bronchiolar epithelium. The presence of mature viral particles within these lesions by electron microscopy confirms that they are sites of productive viral replication. The histologic lesions of sheeppox and goatpox typically include cells with vacuolated nuclei, marginated chromatin, and eosinophilic intracytoplasmic inclusion bodies referred to as sheeppox cells, which represent virus-infected mononuclear phagocytes and fibroblasts. In the lymph nodes and spleen, the essential histological lesion is necrosis and lymphoid depletion (N. James MacLachlan, 2017).
Initial experimental investigation indicated that viremia persisted for about four days. However, in more recent studies that employed more sensitive diagnostic methods, viral genomic material could be detected by PCR for up to eight days. After experimental infection, LSDV was present in skin nodules, lymph nodes, liver, kidneys, saliva, nasal mucosa and semen of infected animals. Skin nodules contain high levels of virus (up to 106 TCID50/ml). High levels and shedding of viruses were also found in the nasal mucosa of affected animals. The virus has been demonstrated electron microscopically in keratinocytes in the epidermis, and fibroblasts and endothelial cells and pericytes of blood vessels in the dermis in affected parts of the skin. Immunohistochemistry revealed that several different cell types may contain LSDV antigen, including keratinocytes, hair follicle epithelium, and fibroblasts and macrophages in the dermis, subcutis and lymph nodes (Coetzer et al., 2018).
Animals that have recovered from natural infection by the virus have shown lifelong immunity. Calves from their infected dams are resistant to clinical disease for approximately six months because of the acquired maternal antibodies. Affected animals clear the infection and no carrier state has known for LSD virus yet (Tuppurainen et al., 2017).
6. Clinical signs and pathology of LSD
Lumpy skin disease signs range from inapparent to severe disease. There is no current evidence of variation in virulence regarding the different LSDV strains. The characteristic nodular skin lesions appear on the head, neck, chest, abdomen, perineum, genitalia, udder and limbs. The center of the lesion often ulcerates and with time a scab forms on top (Tuppurainen et al., 2017). The incubation period in naturally infected animals may be up to 28 days. Clinical signs in cattle, besides the skin nodules, include lachrymation, nasal discharge, high fever (>40.5°C), appetite loss, enlarged subscapular and femoral lymph nodes, sharp drop in milk yield, necrotic plaques in oral and nasal mucous membranes and reduced fertility. Buffaloes may also show skin lesions (Roche et al., 2021).
Once scabs are found, the virus has probably been circulating within the herd for at least 3–4 weeks. LSDV is present in the skin lesions and the scabs, blood, nasal, oral and ocular secretions, semen, and sometimes in the skin of cattle without visible clinical signs. A study showed that only half of the experimentally infected cattle develop skin lesions. Non-clinical but viraemic animals are common and maybe a source of infection through vectors such as mosquitos that feed directly on small blood vessels or spread the disease when moved by foot or in a vehicle. Infected animals shed the virus through oral and nasal secretions that may contaminate common feeding and water troughs. Experimental studies confirmed virus transmission through artificial insemination and the negative impact of LSDV contaminated semen on in vitro fertilization (Annandale, 2020).
7. Diagnosis
7.1 Differential diagnosis
Severe cases of LSD are highly characteristic and easy to recognize, but early stages of infection and mild cases may be difficult to distinguish even for the most experienced veterinarians, requiring a laboratory confirmation. Samples should be collected from all suspected animals and tested using fast and highly sensitive PCR methods to differentiate true cases. The following diseases may be considered as a differential diagnosis for LSD:
- Pseudo lumpy skin disease/Bovine herpes mammillitis (bovine herpesvirus 2): dermal lesions may look like those caused by LSDV, but are more superficial and the course of the disease is shorter and less severe. The disease can be ruled out by detecting LSDV by PCR.
- Insect bites, urticaria, and photosensitization: dermal lesions may look like those caused by LSDV, but are more superficial and the course of the disease is shorter and less severe. The disease can be ruled out by detecting LSDV by PCR.
- Pseudocowpox (Parapoxvirus): lesions occur only on the teats and udder. The disease can be ruled out by detecting LSDV by PCR.
- Dermatophytosis: early ringworm lesions, more superficial, clearly different, the non-ulcerative surface structure of the ringworm lesion.
- Demodicosis: dermal lesions predominantly over withers, neck, back, and flanks, often with alopecia present. The disease can be ruled out by the detection of mites using skin scrapings.
- Bovine papular stomatitis (Parapoxvirus): lesions occur only in the mucous membranes of the mouth. The disease can be ruled out by PCR testing.
- Besnoitiosis: lesions often occur in the scleral conjunctiva, and dermal lesions may exhibit alopecia with thick and wrinkled skin. The disease can be ruled out by detecting LSDV by PCR.
- Onchocerciasis: dermal lesions most likely at the ventral midline. The disease can be ruled out by PCR.
7.2 Laboratory diagnosis
Lumpy skin disease virus was identified in seven cows by a gel-based PCR (Ireland and Binepal, 1998). Virus isolation and electron microscopy were performed on excised skin lesions as described by Brenner et al (Brenner et al., 2006). Following virus identification in the first cases, the diagnosis was mostly performed based on clinical signs according to the case definition described in the previous section.
- Identification of the agent
· Polymerase chain reaction (PCR) is the least expensive and quickest method for detection of LSD virus. Skin nodules and scabs, saliva, nasal secretions, and blood are suitable samples for PCR detection of LSD virus.
· Virus isolation followed by PCR to confirm the virus identity takes longer and is more expensive but has the advantage of demonstrating the presence of live virus in the sample.
· Electron microscopy can be used to identify the classic poxvirus virion but cannot differentiate to genus or species level.
- Serological tests
It is not possible to distinguish the three viruses in the Capripoxvirus genus using serological techniques.
· Virus neutralization: this is currently the gold standard test for the detection of antibodies raised against Capripoxviruses.
· Western blot: highly sensitive and specific but expensive and difficult to perform.
· Capripoxvirus antibody enzyme-linked immunosorbent assay: new commercial kits for detection of capripoxvirus antibodies are currently being developed and released on to the market.
8. Treatment
LSD is caused by a virus which means that there is no specific treatment. Control of LSD must focus on prevention, including vaccination.
Animals are usually treated using supportive therapy of local wounds to prevent fly infestation and secondary infections. Systemic antibiotics may be given for more serious cases of the disease. The animals may become debilitated for up to six months, with a drop in milk production, caused by loss of feed intake due to mouth lesions. Mobility and fertility can also be impacted. Under pastoral conditions, animals may become dehydrated and starve to death. Secondary bacterial infections of skin lesions are common and pneumonia may be a complication in animals with mouth lesions (Roche et al., 2021).
VEMEDIM please introduce some methods to prevent Lumpy Skin Disease.
1. Product to kill flies
- Fly killer: 50-100g/ 100 m2
- Fronil Spot to kill ticks on cattle’s skin: 5ml/head
2. Disinfectant products:
Disinfectant products | Spray inside the animal barns | Spray outside the animal barns | Spray on the aceration, wound, dermatitis and footrot. |
15ml/ 4 liter of water |
| 10ml/ 2 liter of water | |
Disina | 3.3 ml/ liter of water | 5 ml / liter of water |
|
10ml/ liter of water | 20 ml/ liter of water |
| |
|
| Spray on the ulcer area | |
|
| Spray on the ulcer area |
3. Drugs treat secondary infection
- Antibiotics: + Ceftiofen 1 ml/45kg of body weight, one time/ day, 5-7 consecutive days
+ Azi fluxin 1ml/ 20kg of body weight, one time/day, 5-7 consecutive days ( in case cattle show the sign related to respiration problems as cough, runny nose, …
- Inflammation: + Chymosin fort 1ml/ 30-40 kg, one time/day, 3-5 consecutive days
+ In case, cattle is getting pernant, need to be supplied Progesteron 5-10 ml/ head
9. Control and prevention of disease
9.1 Control and prevention
In each country and region where LSD outbreaks occur, the control and eradication measures need to be adjusted to local settings, taking into consideration the size of the susceptible cattle population, local cattle farming practices, LSD risk factors and social and religious traditions and beliefs. These regionally specific factors will determine which control and eradication measures are feasible and/or realistically implemented.
Countries need to decide on achievable, feasible policy goals, become involved in a regional discussion, and ensure that national policy goals are compatible with each other and harmonize control measures.
In general LSD control is based on: 1) vaccination of susceptible populations with >80 percent coverage; 2) movement control of cattle and buffalo and quarantine; 3) biosecurity and vector control; 4) strengthening active and passive surveillance; 5) awareness-raising on risk mitigation among all stakeholders involved; and 6) zoning – large protection and surveillance zones and vaccination zones.
9.2 Vaccination
Vaccination of cattle using a vaccine with demonstrated efficacy is the best option for controlling the spread of LSD, especially if pre-emptive, i.e. applied before the virus enters a region or country at risk. However, preventive vaccination against LSD leads to trade restrictions on the export of live cattle and their products, which may deter disease-free exporting countries from implementing pre-emptive vaccination in high-risk regions. Presently only live, attenuated vaccines are available against LSD virus. There is ongoing research and development of inactivated vaccines. Three groups of vaccines offer good protection against LSDV in cattle: attenuated vaccines based on LSD, SPP or GTP viruses. Vaccines should be produced under good manufacturing practices and according to OIE standards. Efficacy testing is required before applying the vaccine. Vaccine challenge experiments should be carried out at specialized authorized laboratories only. The vaccine selected for use in a country should meet the recommendations of the International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products program and should comply with the regulatory approval procedure of the country. In addition, vaccines should be manufactured by the principles set out in the OIE standards for veterinary vaccines/biologicals.
Cattle are protected from vaccinations derived from sheep or goats because all strains of Capripoxviruses share a major neutralizing site. Theoretically, inoculation with one strain leads to immunity against all others. In practice, cattle vaccination with sheep and goat pox strains leads to insufficient protection, so they are only used in countries where sheep and goat pox are endemic. Since sheep and goat pox have not occurred in South Africa, only the LSD virus strain vaccine is used in this region. There are no vaccines or tests to differentiate infected from vaccinated animals (DIVA) (Panel, 2015).
The OIE recommends that when using a vaccine meant for sheep or goats, it should first be tested on the most susceptible breeds in peak lactation. Capripoxvirus vaccines cause a visible reaction at the inoculation site in Bos taurus. The risk for LSD virus outbreaks in herds with these species can be much higher, as many owners refuse to vaccinate their animals because of this side effect (Zeynalova et al., 2016).
10. References
Alexander, R., Plowright, W., Haig, D., 1957. Cytopathogenic agents associated with lumpy skin disease of cattle. Bulletin of epizootic diseases of Africa 5, 489-492.
Annandale, C.H., 2020. Reproductive effects of lumpy skin disease virus in cattle. Utrecht University.
Annandale, C.H., Holm, D.E., Ebersohn, K., Venter, E.H., 2014. Seminal transmission of lumpy skin disease virus in heifers. Transboundary and emerging diseases 61, 443-448.
Annandale, C.H., Irons, P.C., Bagla, V.P., Osuagwuh, U.I., Venter, E.H., 2010. Sites of persistence of lumpy skin disease virus in the genital tract of experimentally infected bulls. Reproduction in domestic animals 45, 250-255.
Brenner, J., Haimovitz, M., Oren, E., Stram, Y., Fridgut, O., Bumbarov, V., Kuznetzova, L., Oved, Z., Waserman, A., Garazzi, S., 2006. Lumpy skin disease (LSD) in a large dairy herd in Israel, June 2006. Israel Journal of Veterinary Medicine 61, 73.
Brucellosis, O., 2019. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2019, OIE. World Health Organization for Animal Health: Paris, France, 355-398.
Coetzer, Tuppurainen, E., Babiuk, S., Wallace, D., 2018. Lumpy Skin Disease.
Davies, F., Otema, C.J.R.i.v.s., 1981. Relationships of capripox viruses found in Kenya with two Middle Eastern strains and some orthopox viruses. 31, 253-255.
Davies, G.F., 1991. Lumpy skin disease of cattle: A growing problem in Africa and the Near East. FAO Corporate Document Repository. Rome.
EFSA, 2020. Lumpy skin disease epidemiological report IV: data collection and analysis, EFSA J, p. e06010.
Givens, M., 2018. Risks of disease transmission through semen in cattle. Animal 12, s165-s171.
Gupta, T., Patial, V., Bali, D., Angaria, S., Sharma, M., Chahota, R., 2020. A review: Lumpy skin disease and its emergence in India. Veterinary Research Communications, 1-8.
Ireland, D., Binepal, Y., 1998. Improved detection of capripoxvirus in biopsy samples by PCR. Journal of virological methods 74, 1-7.
Irons, P., Tuppurainen, E., Venter, E., 2005. Excretion of lumpy skin disease virus in bull semen. Theriogenology 63, 1290-1297.
Markey, B., Leonard, F., Archambault, M., Cullinane, A., Maguire, D., 2013. Clinical veterinary microbiology e-book. Elsevier Health Sciences.
Mulatu, E., Feyisa, A.J.J.V.S.T., 2018. Review: Lumpy skin disease. 9, 1-8.
N. James MacLachlan, E.J.D., 2017. Chapter 7 - Poxviridae in Fenner's Veterinary Virology, in: MacLachlan, N.J., Dubovi, E.J. (Eds.), Fenner's Veterinary Virology (Fifth Edition). Academic Press, Boston, pp. 157-174.
OIE, 2019. Lumpy Skin Disease. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2019. 355-398.
Panel, E.A., 2015. Scientific opinion on lumpy skin disease. EFSA J 13 (1): 3986, 73 pp.
Roche, X., Rozstalnyy, A., TagoPacheco, D., Pittiglio, C., Kamata, A., Beltran Alcrudo, D., Bisht, K., Karki, S., Kayamori, J., Larfaoui, F., 2021. Introduction and spread of lumpy skin disease in South, East and Southeast Asia: Qualitative risk assessment and management. Food & Agriculture Org.
Tageldin, M.H., Wallace, D.B., Gerdes, G.H., Putterill, J.F., Greyling, R.R., Phosiwa, M.N., Al Busaidy, R.M., Al Ismaaily, S.I., 2014. Lumpy skin disease of cattle: an emerging problem in the Sultanate of Oman. Tropical animal health and production 46, 241-246.
Tuppurainen, E., Alexandrov, T., Beltran-Alcrudo, D., 2017. Lumpy Skin Disease Field Manual-A Manual for Veterinarians; FAO Animal Production and Health Manual No. 20. Rome.
Tuppurainen, E.S., Venter, E.H., Coetzer, J.A., Bell-Sakyi, L., 2015. Lumpy skin disease: attempted propagation in tick cell lines and presence of viral DNA in field ticks collected from naturally-infected cattle. Ticks and tick-borne diseases 6, 134-140.
Weiss, K., Gard, S., 2013. Cytomegaloviruses. Rinderpest virus. Lumpy skin disease virus. Springer.
Yeruham, I., Nir, O., Braverman, Y., Davidson, M., Grinstein, H., Haymovitch, M., Zamir, O., 1995. Spread of lumpy skin disease in Israeli dairy herds. Veterinary Record 137, 91-91.
Zeynalova, S., Asadov, K., Guliyev, F., Vatani, M., Aliyev, V., 2016. Epizootology and molecular diagnosis of lumpy skin disease among livestock in Azerbaijan. Frontiers in microbiology 7, 1022.
* Nguyen Thanh Lam, DVM., MSc., PhD
Department of Veterinary Medicine, College of Agriculture, Can Tho University
Address: Campus II, 3/2 street, Ninh Kieu district, Can Tho city, Viet Nam
Phone: +84 (0) 939-468-525
Email: ntlam@ctu.edu.vn


